Adverse Outcome Pathways and Harm Reduction
What are adverse outcome pathways?
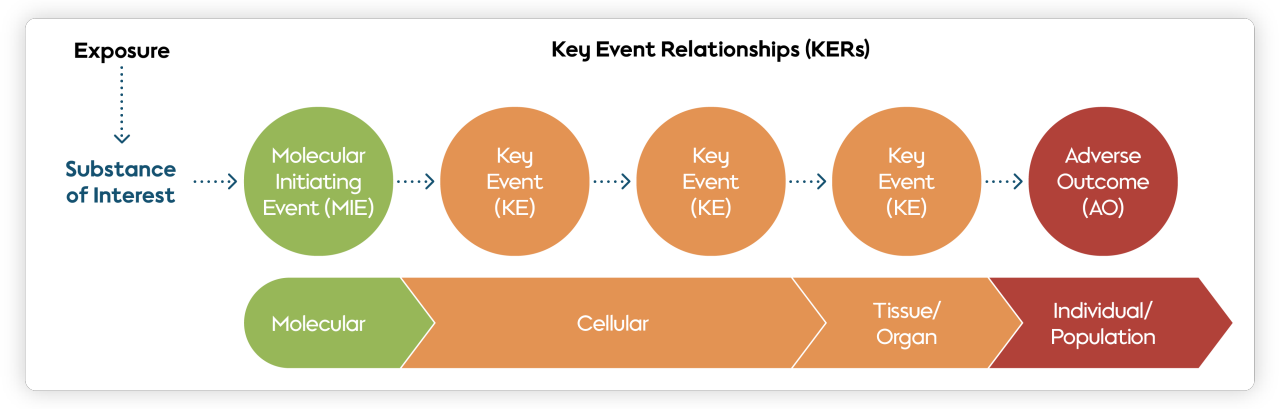
AOPs are simply a way of organizing existing knowledge; they are conceptual frameworks that identify the chain of events taking place on a molecular and cellular level, leading up to adverse effects seen in a living organism or whole population.
At its most simple, an AOP is composed of three elements:
- Molecular initiating event (MIE). An event known as a molecular initiating event instigates a transformation within a biological system. MIEs are brought about by an external factor, usually a chemical—also called a stressor. MIEs reflect the first interaction of the stressor with a cell. Once an MIE has taken place within a biological system, it can set off a cascade of alterations from cellular to tissue or organ level. This sequence of changes can ultimately lead to an outcome in the biological system.
- Adverse outcome (AO). In toxicology, an adverse outcome is a term used to describe a detrimental effect experienced by a living organism as a result of exposure to a stressor. Examples of AOs in toxicology include cancer, impaired organ function, or even death.
- Key event relationship (KER). The series of measurable events taking place within an organism linking an MIE to an AO are known as key events (KE), and the causal relationships between these key events are known as key event relationships (KER). In practice, KEs and KERs connect the MIE from the molecular level all the way to the AO at the individual or even population level. As such, KERs form the heart of the AOP for their predictive applications.
Thanks to these three elements, AOPs offer a pragmatic simplification of the complexity found in biology.
Deriving risk assessment from adverse outcome pathways
AOPs are developed at a level relevant for risk assessment so the knowledge gained can be utilized to support risk-based decision-making. Thus, AOPs can move us away from relying solely on empirical observations, such as in vivo studies, and instead, enable scientists to connect laboratory test results, such as in vitro assays, directly to KEs and KERs and derive consequences at the individual and population level. This leads to better predictive computational models based on our understanding of underlying mechanisms; thus, enhancing our ability to prevent harm. As long as we can understand the initial exposure scenario and subsequent events with great accuracy, AOPs allow us to derive risk estimates.
In fact, in the field of toxicology, the application of AOPs is recognized as an integral component of new approach methodologies to supply data on chemical dangers and risk evaluation without resorting to animal experiments. This highlights their importance in modern practices, also referred to as Next-Generation Risk Assessment.
In risk evaluations, it is crucial to understand the complexity and probable interconnectedness of AOPs. AOPs can be extensively interconnected, giving rise to AOP networks. For example, a single stressor such as a chemical has the potential to initiate several MIEs. This chemical can thus lead to a series of different chains of events, and ultimately, impact an individual in various ways. Therefore, it is important for the risk assessor to understand the circumstances under which each molecular event is initiated. An additional illustration of the influence of AOP networks might be that a single MIE could trigger a series of events due to the interlinked nature of KEs and KERs, leading to varied AOs.
Constructing an adverse outcome pathway
Recognizing the importance of the interconnectedness among biological pathways, it was imperative to establish a shared repository for managing AOPs. With this in mind, the Organization for Economic Cooperation and Development (OECD) launched the Adverse Outcome Pathway Knowledge Base (AOP-KB) which plays a pivotal role “[t]o enable the scientific community, in one central location, to share, develop and discuss their AOP related knowledge.”
Using this collaborative tool, scientists can build AOPs by entering and then linking information about the MIEs, KEs, KERs, and AOs. This helps avoid unnecessary duplication and facilitates the accumulation of shared knowledge.
The AOP-KB comprises four independently developed platforms: AOP-Wiki, Effectopedia (in Beta), AOPXplorer, and Intermediate Effects DB1 (in development). Each platform emphasizes different types of captured information, providing a comprehensive and diverse knowledge base, and share, exchange, and synchronize information via the AOP-KB hub.
When constructing an AOP, scientists often rely on the AOP Developers’ Handbook which takes a stepwise approach:
- Exposure understanding: Know how the exposure (chemical, physical, or biological) interacts with cellular components. Understand its impact on molecules, proteins, and organelles.
- Outcome definition: Envision the individual’s response post-exposure. Is it a disease endpoint, a clinical outcome, or a specific adverse effect? Define the outcome.
- Bridging the gaps: By using the handbook and studying the literature, connect exposure to outcome, detailing the sequence within the chain of events.
The AOP development is guided by an AOP coach assigned by OECD. The AOP coach helps authors adhere to the handbook and guidance and that all required information is provided. There is also a choice of opening the AOP on the AOP-Wiki for public comment, allowing the authors to incorporate the feedback as applicable. Once the AOP is finalized and the AOP coach has made sure that all the relevant steps have been taken, the AOP is ready for the review process.
There are two options for review, both of which are considered independent; either a call for reviewers is made or the review can be done through peers via a scientific publication(s). Either way, the reviews will be overseen by the Extended Advisory Group on Molecular Screening and Toxicogenomics (EAGMST) which is an advisory group under the Working Group of the National Coordinators for the Test Guidelines Program (WNT). Once EAGMST is satisfied by the peer review, they will recommend AOP endorsement to WNT, and the AOP can be used for toxicological risk assessment by anybody in the field.
Notably, due to their interconnectivity, the use of AOPs extends beyond human health; disciplines such as ecotoxicology or climate change recognize their value as AOPs can guide conservation efforts by pinpointing critical events that threaten species survival.
PMI’s contribution to adverse outcome pathways
As part of our scientific research, we have been actively contributing to the development of AOPs which can be used to deduce the potential for harm reduction of our smoke-free products.
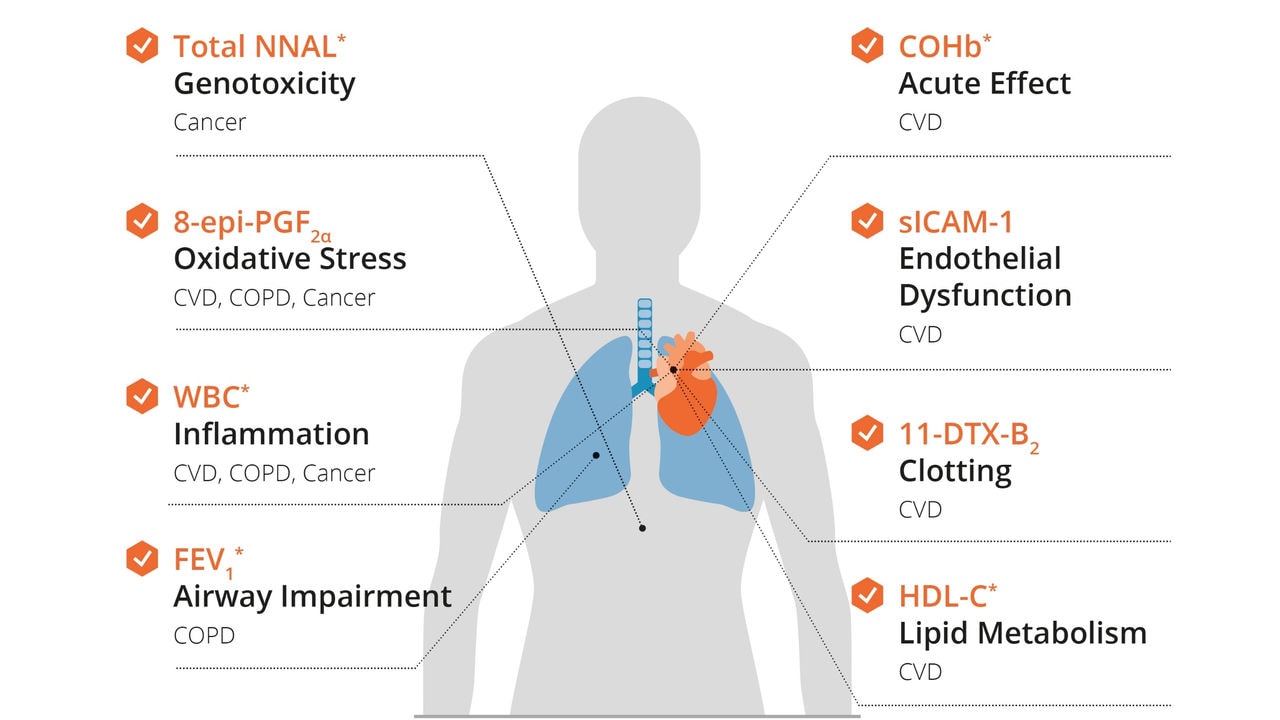
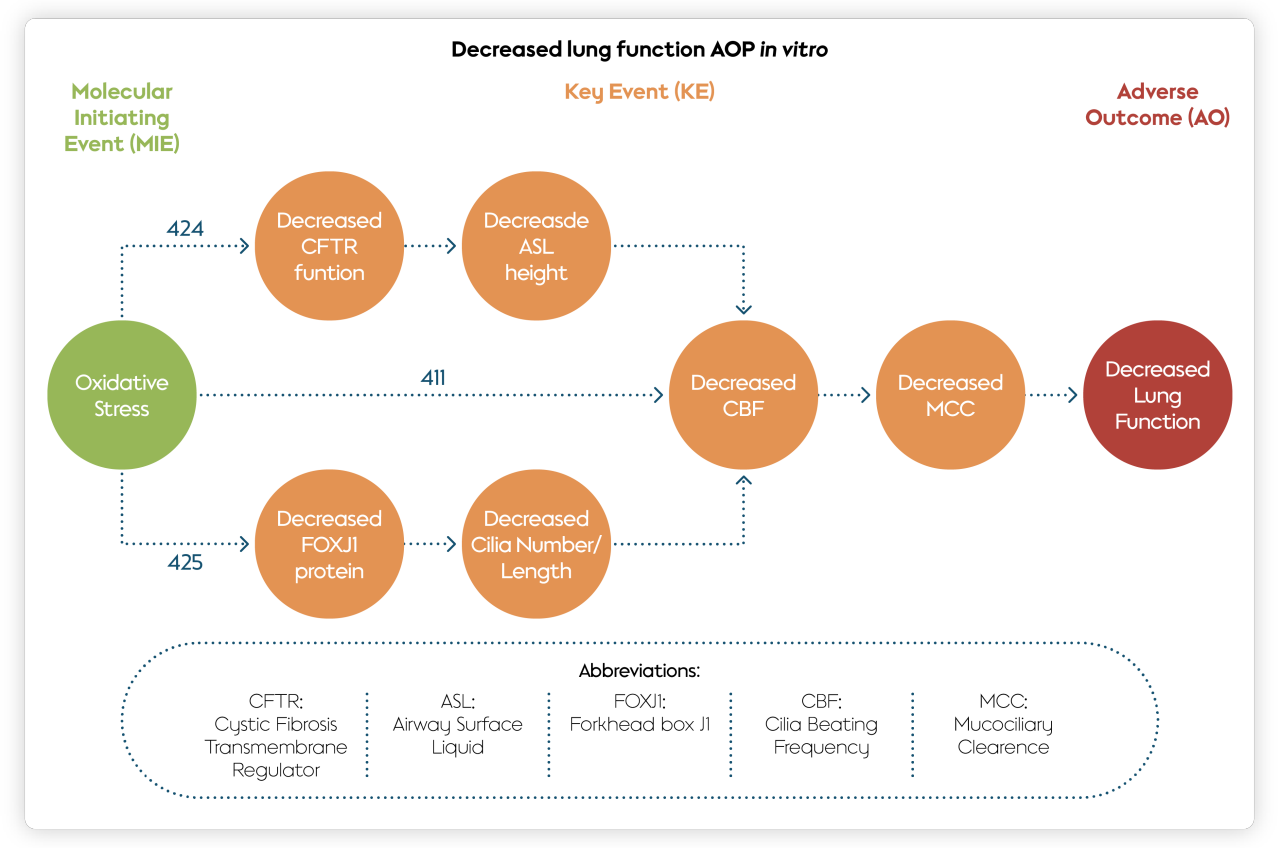
For example, we and our external partners have documented three AOPs (411, 424, and 425) showing how an MIE derived from cigarette smoking—oxidative stress—leads to decreased lung function. These AOPs focus primarily on a condition known as impaired mucociliary clearance, where the self-clearing mechanism of the airways is not functioning properly. A decrease in mucociliary clearance can lead to chronic obstructive pulmonary disease and asthma. More insights on how oxidative stress impairs mucociliary clearance can be found in this publication.
It is worth noting that AOP 411 is the first example of using a quantitative AOP to enable predictions of probability or severity of adverse outcomes from the use of tobacco products. Researchers combined data from an advanced in vitro organotypic airway model exposed to cigarette smoke or tobacco heating system (THS) aerosol with an AOP for increased oxidative stress. The modelling predicted diminished reduction in lung function in response to the use of the THS, our leading heat-not-burn product, compared with continued smoking. This current approach may also present a basis for an integrated approach to testing and assessment of tobacco products for future regulatory decision-making.
The three AOPs are linked as follows:
In a separate publication, also written with external partners and centered on lung-related pathways, we have elaborated AOP 148. This pathway demonstrates how smoking-induced oxidative stress can activate the Epidermal Growth Factor Receptor (EGFR) in the respiratory system. EGFR plays a crucial role in cell signaling pathways that regulate cell division, which can lead to an increased production of mucus, resulting in impaired lung function.
Still working with external partners, we have studied an AOP composed of eight KEs detailing how peptide oxidation, an MIE triggered by chronic chemical exposure like those found in cigarette smoke, can lead to hypertension.
Adverse outcome pathways and the tobacco harm reduction approach
These examples showcase how AOP research supports product assessment and the harm reduction approach. This strategy provides a public health viewpoint, offering an intuitive way to think about how better alternatives to a harmful behavior, and an increased rate of adopting these better alternatives, can benefit public health even if these alternatives do not eliminate risk of harm.
In the case of smoking, scientifically substantiated better alternatives to conventional tobacco products such as smoke-free products, have the potential to present less risk of harm for adults who would otherwise continue to smoke. Thus, by utilizing AOPs to evaluate the reduced risk associated with the use of smoke-free products, we can add valuable insights for regulators enabling their informed decision-making process with regard to tobacco harm reduction and smoke-free products.