ABOUT™ – Health and Functioning
What is the ABOUT™ – Health and Functioning instrument?
The ABOUT™ Toolbox has been created to evaluate and assess consumers’ perception and behavior regarding tobacco and nicotine product (TNP) use. It includes various Consumer Reported Outcome Measures (CROM) designed to support research on a broad range of TNPs, as well as regulatory submissions and modified risk tobacco product (MRTP) applications.
Existing self-reported measures for evaluating perceived health and quality of life are often not sensitive enough to assess the impact of TNPs on the perceived health and functioning status of users. The Health and Functioning instrument has been developed to accurately reflect the perceived health and functioning status of individuals who use TNPs, with a particular focus on detecting subtle changes in adult smokers who switch to smoke-free products. Since February 2018, we have worked on the Health and Functioning instrument, aiming to make it a comprehensive tool for the evaluation of the impact of smoke-free products on individual and public health.
Theoretical framework and main development phases
The development of the instrument has been underpinned by theoretical conceptual frameworks such as: the World Health Organization’s (WHO’s) International Classification of Functioning, Disability, and Health (ICF); the revised Wilson and Cleary Model of health-related quality of life; and the U.S. Food and Drug Administration’s (FDA’s) guidance on patient-reported outcome (PRO) measures. Based on these and other frameworks, the development process of the new self-report instrument was organized into the following three main phases.
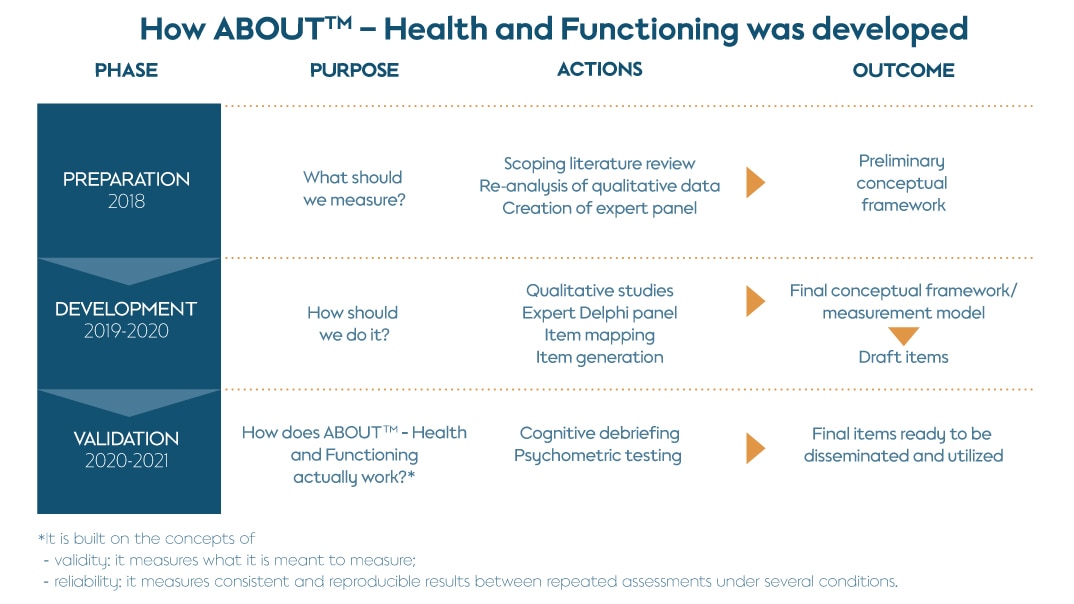
A. PREPARATION (2018)
This phase incorporated background research and consultations with subject matter experts to guide the selection of methodologies for identifying relevant concepts. It included a comprehensive scoping literature review of 97 existing publications, aiming at identifying both the positive and negative impacts that TNP use could have on users’ health and functioning. This was followed by a re-analysis of focus group data from the development of the ABOUT™ – Perceived Risk instrument and individual TNP users, in order to extract meaningful preliminary consumer insights on health and functioning.
Additionally, a panel of experts, combining expertise in tobacco science, PRO measures, regulatory science, and health economics provided guidance in all phases of development of the instrument. From this preparatory phase, key health and function domains impacted by TNP use were identified, including physical health signs and symptoms, general physical appearance, functioning (physical, sexual, cognitive, emotional, and social), and general health perceptions.
Following this, a preliminary conceptual model was developed to identify domains that would be included in the instrument.
B. DEVELOPMENT (2019-2020)
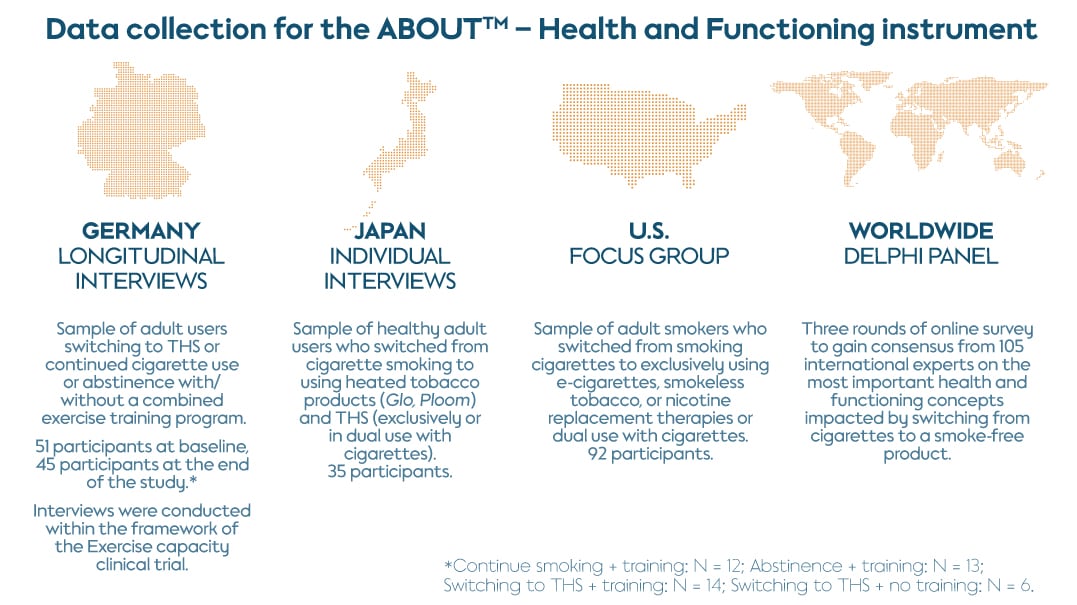
This phase used both qualitative and quantitative methodologies to develop suitable items to capture information in the identified domains. First, we conducted a clinical study on exercise capacity in Germany, where we included longitudinal interviews with 45 adult participants who had switched to the Tobacco Heating System (THS), continued cigarette use, or abstained, with or without a combined exercise training program.
We then carried out individual interviews in Japan to explore changes in perceived health and functioning with a sample of 35 healthy adults who switched from cigarettes to THS and other smoke-free products (exclusively or dual use with cigarettes).
Finally, we conducted 10 focus groups with adult smokers in the U.S. who switched from cigarettes to e-cigarettes, smokeless tobacco, or nicotine replacement therapies (exclusively or dual use with cigarettes). These qualitative studies allowed the identification of relevant concepts and draft item generation for the instrument related to the health and functioning status experienced by adult users switching from cigarettes to smoke-free products.
Following these three rounds of qualitative studies, we recruited a panel of 105 international clinical and healthcare experts for a global Delphi panel survey. This sought to identify the most important concepts to measure when evaluating health and functioning status associated with switching to smoke-free products and ensure the clinical and cross-cultural relevance of the instrument.
C. VALIDATION (2020-2021)
Research activities to support validation of the ABOUT™ – Health and Functioning measure included qualitative cognitive debriefing interviews to assess appropriateness, understanding, content validity of the instrument, and guide refinement of the items. A quantitative validation survey was then conducted with current users of different TNPs (cigarettes, e-cigarettes, heated tobacco products, smokeless tobacco products, nicotine replacement therapy) in the U.S., Japan, Germany, Italy, and Russia. This assessed psychometric properties, reliability, and validity of the measure and provided supportive evidence for its key measurement properties.
The ABOUT™ – Health and Functioning instrument currently comprises 10 different scales (146 items) covering different health (i.e., coughing breathing, lung health, breathing difficulty, oral health, general health, throat and voice, appearance, and general health) and functioning concepts (i.e., working life, social functioning, wellbeing, and bother with smell). Each scale is completed by a set of global items assessing severity of the impact of TNP use and change since switching from cigarettes to another TNP.
Applications for the ABOUT™ – Health and Functioning instrument
The ABOUT™ – Health and Functioning instrument is designed to provide a sensitive and comprehensive measure of perceived health and functioning status, offering valuable insights into the nuanced impacts of TNP use on individual well-being. By filling existing gaps in smoking-related quality of life measures, it enhances research efforts, informs regulatory decisions, and supports consumer understanding. The data generated will facilitate more informed choices and contribute to better public health management and the advancement of tobacco harm reduction strategies.
The ABOUT™ – Health and Functioning instrument was administered in a recently completed clinical study, conducted by PMI, which examined the reduction in toxicant exposure, inflammation, and oxidative stress in adults who used THS exclusively for 2 years, compared with those who continued smoking cigarettes. In the study, the instrument was administered as a TNP-specific health status measure to determine whether aspects including shortness of breath during daily activities, coughing, other respiratory symptoms, and general health status were impacted by TNP use. The findings suggest that THS use may offer self-reported health and functioning benefits compared with continued cigarette smoking.
In the future, PMI researchers will be exploring the further development of the ABOUT™ – Health and Functioning instrument. This will include using data collected with the instrument to further inform its psychometric properties, especially when applied in a longitudinal setting to evaluate the impact of switching from cigarettes to smoke-free products on health and functioning status over time. Additionally, further testing will be utilized to continually refine the instrument measurement properties and scoring, and to facilitate efficient, accurate, engaging, and tailored administration of the measure.

Read the Scientific Update magazine
The Scientific Update magazine is focused on PMI's research and development efforts, milestone studies, industry regulations, and more. View the latest issue, or read the articles online.


